Background: Extranodal Natural Killer/T Cell Lymphoma (ENKTL) is a highly aggressive form of non-Hodgkin lymphoma (NHL), primarily found in Asia and commonly linked to Epstein-Barr virus (EBV) infection. Despite the treatment efficacy for early stage ENKTL has been improved in recent years, there is no consistent approach for clinical treatment. Current guidelines includes radiotherapy (RT) alone, concurrent chemoradiation therapy (CCRT), sequential chemoradiation, and sandwich chemoradiation. Here we present the final results of the efficacy and safety of sequential therapies of DDGP (dexamethasone, cisplatin, gemcitabine, and pegaspargase) regimen or VIPD (etoposide, ifosfamide, cisplatin and dexamethasone) regimen combined with RT, and RT alone.
M ethods: We conducted a prospective, multicenter, randomized, controlled clinical trial to compare the efficacy and safety of sequential chemotherapy (DDGP vs VIPD regimen) followed by RT, sequential RT followed by chemotherapy, and RT alone in eligible patients with newly diagnosed stage I/II ENKTL. Upon study entry, patients were randomized 1:1:1:1:1 to receive either DDGP regimen followed by RT (DDGP+RT), or VIPD regimen followed by RT (VIPD+RT), or RT followed by DDGP regimen (RT+DDGP), or RT followed by VIPD (RT+VIPD), or RT alone. Patients assigned to the DDGP group were administered cisplatin (20 mg/m 2, intravenous, days 1-4), dexamethasone (15 mg/m 2, intravenous, days 1-5), gemcitabine (800 mg/m 2, intravenous, on days 1 and 8), and pegaspargase (2500 IU/m 2, intramuscular, on day 1). Those in the VIPD group received etoposide (100 mg/m 2, intravenous, days 1-3), ifosfamide (1.2 g/m 2, intravenous, days 1-3), cisplatin (33 mg/m 2, intravenous, days 1-3), and dexamethasone (40mg, intravenous, days 1-4). Both the DDGP and VIPD regimens were repeated every 21 days. For the sequential chemotherapy followed by RT group, patients underwent 3 cycles of either DDGP or VIPD regimen, followed by 50 Gy (2 Gy * 25 times) of intensity modulated radiotherapy (IMRT). In the sequential RT followed by chemotherapy group, patients were first given 50 Gy (2 Gy * 25 times) of IMRT and then sequential either DDGP or VIPD regimen. Meanwhile, the RT alone group received 50 Gy (2 Gy * 25 times) of IMRT only. The primary end point of our study was progression free survival (PFS), with secondary end points including complete remission rate (CRR), objective response rate (ORR), overall survival (OS).
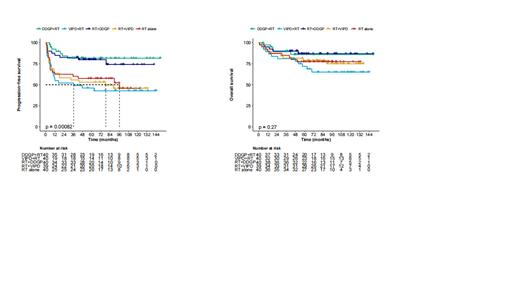
R esults: Our study, spanning January 2011 to August 2020, involved 244 patients from 22 centers across China, with 200 randomly assigned to receive treatment (40 in each group). Both the demographic and disease characteristics of the patients at baseline were generally consistent across all treatment groups. During a median follow-up of 72.1 months, the median PFS (mPFS) was not reached in neither the DDGP + RT nor RT + DDGP groups, compared to 36.3 months in the VIPD + RT group, 78.3 months in the RT + VIPD group, and 89.6 months in the RT alone group. The 5-year PFS rates for the DDGP + RT, VIPD + RT, RT + DDGP, RT + VIPD, and RT alone groups were 81.5%, 46.1%, 79.8%, 53.1%, and 57.5% respectively. Similarly, the 10-year PFS rates were 81.5%, 42.8%, 74.1%, 45.9%, and 45.7% respectively. Compared with RT alone, PFS was improved in DDGP + RT group (p = 0.006) and RT + DDGP group (p = 0.024). Compared with VIPD + RT, PFS was improved in DDGP + RT group (p = 0.006) and RT + DDGP group (p = 0.026). Either between DDGP +RT and RT + DDGP group or between VIPD +RT and RT +VIPD group, PFS showed no differences. Median OS was not reached in 5 groups. OS showed no differences in 5 groups (p = 0.27). The patients who failed from either VIPD regimen or RT alone received DDGP regimen. This might be the reason why the OS showed no differences between 5 groups. The CRRs of 5 groups (DDGP + RT, VIPD +RT, RT + DDGP, RT +VIPD, RT alone) were 87.5%, 57.5%, 82.5%, 69.2%, and 67.5%, respectively. Similarly, the ORRs were 97.5%, 77.5%, 90.0%, 74.4%, and 87.5%, respectively.
Conclusions: The DDGP regimen exhibited promising outcomes in this randomized clinical trial for newly diagnosed early-stage ENKTL patients. The sequential therapies with DDGP regimen could improve the efficacy compared with RT alone. Patients could also benefit more from therapies with DDGP regimen than those with VIPD regimen. The order in which chemotherapy and RT were applied has no fatal impact on the prognosis of early-stage ENKTL.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal